What is the FDA committee deciding?
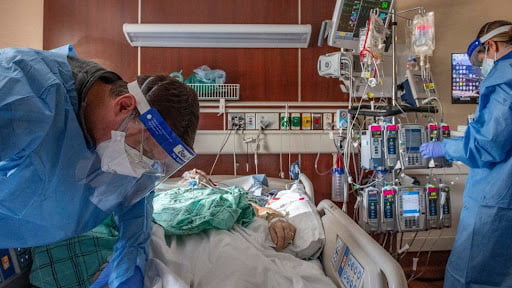
On Friday, a federal advisory committee will decide whether a third shot of the COVID-19 vaccine is safe and protective against infection.
The third shot is a luxury rather than an essential part of complete protection against the President-President’s virus. Anthony Fauci said this week. But he and other federal officials, including President Joe Biden, believe it is time to fire the shot to cover what seems to be waning protection.
In a briefing on the information provided to the committee and posted online on Wednesday. The FDA points out that the studies arguing for a booster were inconclusive and were not conducted in the United States, where the results differed from those in other countries. Overall, the data suggest that COVID-19 vaccines are licensed and approved in the US. The FDA report said they offer some protection against severe Covid-19 disease and deaths in the UK. In the UK, about 90% of people hospitalized with Covids 19 were unvaccinated. Therefore, at a White House briefing last week, some vaccinated people started to catch milder forms of the virus, said CDC Director Dr. Rochelle Walensky.
Committee Members Approval
If the committee members, experts in all vaccine development and immunology, decide that the third dose is safe and effective. It is up to the Centers for Disease Control and Prevention and its advisory committee, which meets on Wednesday and Thursday. Thus, to decide whether to receive a booster dose as planned six months after the first dose. It is not clear whether the committee will support the booster dose. The CDC is considering the Pfizer Biotech vaccine, the only approved vaccine. The Moderna vaccine is still have to review.
On Wednesday morning, Fauci told a class at the Massachusetts Institute of Technology that he thinks people will need three doses of the Moderna and Pfizer Biotech vaccines to obtain adequate long-term protection from COVID-19. Other diseases, such as hepatitis B, require a three-dose vaccine for complete protection. We need to look at that after the dust settles, and it is my opinion and that of many of my colleagues. Although, the proper complete treatment is three-dose therapy, as opposed to two-dose treatment.
Where was the vaccine first initiated?
Israel was one of the first countries to introduce a large vaccine against COVID-19. Israel has some of the best data on how well the vaccine holds over time. The Israeli data cited by Fauci suggest that vaccination protection diminishes over time, starting about six months later. Along with vaccinated people becoming more susceptible to mild and severe diseases.
Israeli officials expect to attend the Advisory Committee on Vaccine-Related Biological Products on Friday to explain their findings in detail. The available data have convinced other public health and vaccine experts.
The purpose of vaccines is not to prevent every single symptom of infection. But to protect people from the worst consequences says Dr. Anna Durbin. She studies experimental vaccines at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “I am convinced that the protection against symptomatic infections is waning,” Dr. Durbins said. “But I am even more convinced that the protection against serious diseases is waning.
“There’s no indication that we don’t need additional boosters,” she said in a phone call with the media on Wednesday. There is no doubt that the boosters are effective.
“We are not wasting precious resources because we realize that it will not be possible to prevent COVID-19 from entering the United States until the virus is stopped elsewhere in the world. In other words, we need the boosters. Hence, President Trump said in his letter that getting the advocates was based on science, not equitable distribution.
In his letter, he pointed out that booster doses are more effective against the highly infectious Delta variant. Therefore, accounting for half of the COVID-19 infections in the US. Studies have shown that adding a third dose to the same vaccine – the same dose as the first two – can increase protection against all virus variants. According to Pfizer and Biotech, the third dose is safer than the two vaccines and has the same side-effect profile.
Study about the new Boosters
Moderna released its data on Wednesday that supports boosters six months ago. Both Pfizer and Biotech have booster shots that are considered for their FDA-licensed vaccines. Moderna and Johnson & Johnson have emergency approval vaccines.
The study, which was not peer-reviewed, followed people from the company’s first vaccine efficacy study in which volunteers received the vaccine or a placebo. A study by Kaiser Permanente in Southern California showed that the vaccine prevented 87% of COVID-19 cases and 96% of hospitalizations. All study participants were exposed to the delta variant.
They were all vaccinated again at the end of the study, meaning they received their vaccinations about a month later than the first group. Of the more than 14,000 people in the group. Those who received their vaccinations earlier were more likely to develop COVID-19 and severe illnesses. Three people who got the earlier shots were hospitalized, and one died. However, suggesting that protection from serious diseases diminishes over time. There is no data on whether people receiving a single J & J vaccine need a second one.